 I’ve realized I need to up my game if I want my blog to get more recognition. I mean, my entries are so cool they have absolute zero notes. (pun and sarcasm intended). I think by now you should have an idea what our experiment is about and the procedure is fairly easy you can try it at home, if you have the materials.
I’ve realized I need to up my game if I want my blog to get more recognition. I mean, my entries are so cool they have absolute zero notes. (pun and sarcasm intended). I think by now you should have an idea what our experiment is about and the procedure is fairly easy you can try it at home, if you have the materials.
I think it is safe to say that everyone knows what temperature is but a common misconception about this property is that it can be measured directly. Actually, the changes in the attributes (thermometric properties) of materials to heat or cold are what really is measured. Two or more objects, with different temperatures, in contact will eventually reach a point where they would have the same temperature. This phenomenon is known as thermal equilibrium. But which one travels faster, hot or cold? The answer is hot, because everyone can catch a cold! Haha. But if you look at it, there might be some truth to this joke. In a previous experiment we did about Sound, we learned that temperature is directly proportional to the speed of sound and exposing a liquid to high temperature increases the speed of the molecules because of the addition of energy in the form of Heat. Thermal sensors are used to measure the heat transferred from an object to or from it. Reaching thermal equilibrium is necessary to get the correct reading. The speed of achieving this depends on the thermal constant, τ , of the sensor given by the equation:
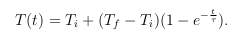 (1)
(1)
where Ti is the initial temperature and Tf is the final temperature. We can linearize this equation to see the relationship between the thermal constant and the amount of time needed.
Linearizing this equation, we get:
(2)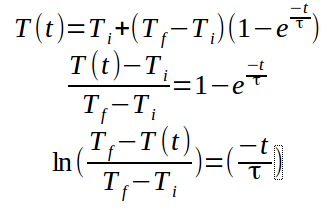
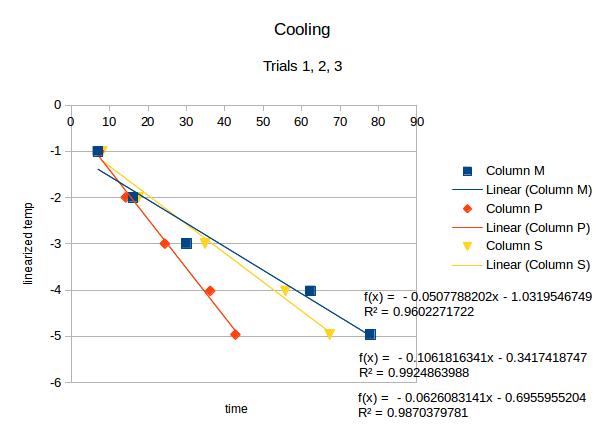
through this equation, we can obtain the value of tau graphically. The slope is equal to (-1/tau).
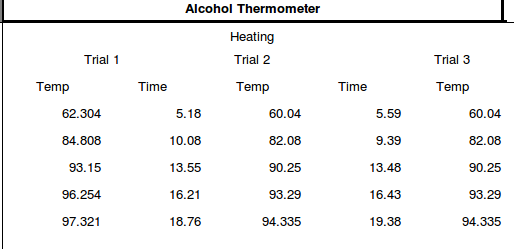
The objectives of the experiment is to operate different temperature sensors, measure the thermal time constant of different temperature sensors, and determine the minimum waiting time before reading measurements of temperature sensors but due to some problems, we were only able to use an alcohol thermometer. But as i said earlier the procedure is easy. The experiment is basically divided into two parts, each conducted thrice; heating and cooling.
Heating:
The thermometer was dipped first on boiling water. Once the reading is constant, the value obtained was set as the final temperature. Then the same thermometer was dipped into ice water and the moment the temperature stays the same is set as the initial temperature.
Cooling:
The thermometer was dipped first in ice water and the final temperature was recorded. Then the thermometer was dipped in boiling water until it stops increasing and the reading was set as the initial temperature.
The following values were obtained:
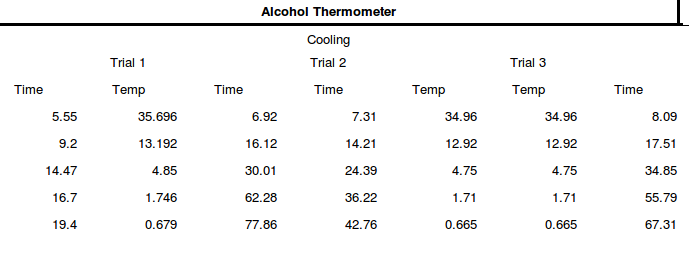
According to eq. 2, here is the graph :
Actually, this experiment may be easy but it wasn’t that fun or informative. Especially since the equipment where not provided, and it is very time consuming and prone to error.